Improving the Rare Disease Diagnostic Journey with Advanced AI
The journey from first symptoms to diagnosis is a long one for most patients with rare diseases. According to a survey from EURODIS, 25 per cent of patients with among the most common rare diseases waited between 5 and 30 years for a diagnosis and 40 per cent were misdiagnosed during that time.
There are many reasons why diagnosis is so challenging. One is that most physicians have limited knowledge about rare or ultra-rare diseases. Another is that as many as 60% of rare diseases present with significant heterogeneity of symptoms, making it extremely difficult to diagnose patients early enough in their disease progression.
A third barrier to diagnosis can often be attributed to the complexity of the diagnostic test currently available. For some rare conditions, deep muscle biopsies are still used for diagnosis and for some rare heart conditions, stents are often the only commercial diagnostic tool available to accurately identify a condition.
In the area of rare immune disorders, there are 349 known unique diseases with more than 800 synonyms, while in rare oncology there are 502 unique rare diseases with more than 1186 synonyms. More than 40 companies are developing therapies to treat rare immune disorders and nearly 250 companies are focused on therapies in rare oncology.
Successfully bringing these products to market, however, depends on finding the right patients for clinical trials. For patients, precision diagnostics is needed to ensure they receive the right targeted treatments for better outcomes.
Good data models combined with artificial intelligence has the potential to dramatically improve the rare disease journey through early and accurate diagnosis by identifying patterns in the data associated with a rare disease and finding patients with those disease patterns.
Joining forces for rare diseases
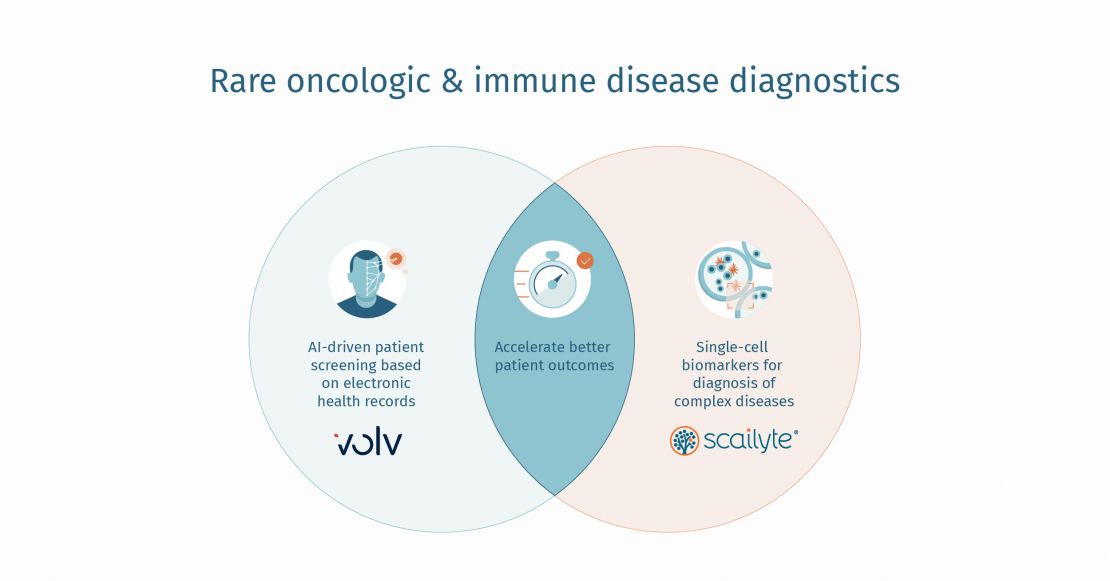
Two companies are approaching the problem of identifying disease biomarkers in different yet corresponding ways. Volv Global identifies digital disease biomarkers by screening patient electronic health records (EHRs) with a unique automated disease learning platform, inTrigue; while Scailyte leverages a single-cell data analysis platform, ScaiVision, to identify and validate disease-specific molecular biomarkers.
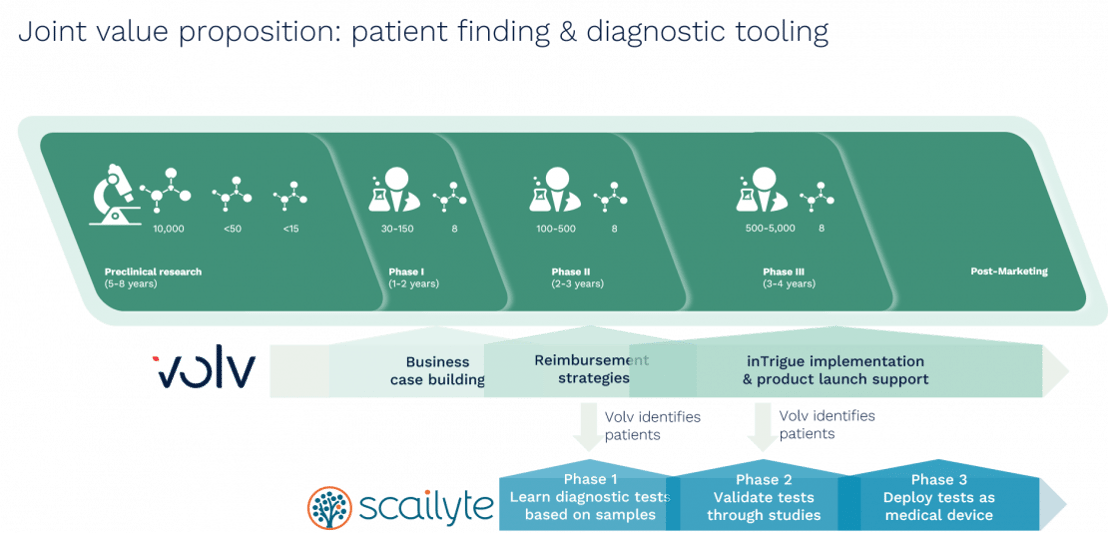
Recognising these important synergies, Volv and Scailyte have joined forces to develop a patient identification and diagnostic platform for rare diseases. In so doing, the two companies are seeking to speed up accurate diagnosis of patients so they can be treated more rapidly, support clinicians with the tools they need to make informed diagnostic decisions and help pharmaceutical companies target their therapies to the right cohort of patients.
Scailyte focusses on oncology, immunology, and immuno-oncology, using its pioneering analytical technology, ScaiVision, to interpret and detect rare events and cells using automated pattern recognition and interpretation of single-cell data. This is achieved by analysing millions of individual cells to identify precise molecular profiles of specific cell types associated with a disease.
As an example, Scailyte developed a diagnostic test for cutaneous T cell lymphoma (CTCL), a rare and severe skin cancer that is hard to diagnose in the initial stages. Using the company’s AI-driven cell analytics pipeline, the company was able to identify CTCL-specific biomarkers that accurately diagnose CTCL with more than 90% specificity and sensitivity. This diagnostic tool will allow for early intervention, which will not only reduce treatment costs but save lives.
Volv Global has adopted a unique approach to identifying undiagnosed or misdiagnosed patients by using a novel algorithm that adds unlabelled or unreliably labelled patient data to the learning procedure. Volv’s InTrigue model learns about a disease through analysis of certain key data points within EHRs, including symptom prioritisation, the patient journey, misdiagnosis, and variance in clinical decision making. It also gathers learnings from: patient registries, clinical trials, market access data, publications, and numerous other sources.
For example, Volv used inTrigue to find undiagnosed patients with acute hepatic porphyria (AHP), finding one patient for every 1.27 flagged. This is a dramatic difference to other standard machine learning methodologies that typically would need a cohort of 50,000 to be confident of a single AHP patient. Importantly, Volv’s predictive diagnostic algorithm actively seeks at-risk individuals rather than waiting for them to become symptomatic.
There is a pressing need to improve the diagnosis of patients with rare diseases and connect these patients with emerging treatments that have the greatest potential to improve and even save lives. Through the partnership, Volv Global will identify potential patients with rare or difficult to diagnose diseases, who can then be enrolled in molecular biomarker discovery studies led by Scailyte and clinical key opinion leaders in the disease area.
Scailyte’s biomarker discovery platform enables diagnosis from cells in body fluids, such as blood and saliva, not only serving to reduce diagnostic complexity as well as cost, but importantly improving the experience for the patient through a non-invasive, patient-centric approach. This, in turn, will empower patients and support their emotional needs as well as their personal needs.
Given the complex nature of rare diseases, the future of accurate, quick, and non-invasive diagnosis lies in an AI and data science approach, built on robust and standardised methodologies.