2022 07 WODC USA Workshop - How can AI inform better Clinical Development strategy, design, and patient stratification?

Credit Photo by Pietro Jeng Selfors on Pexels
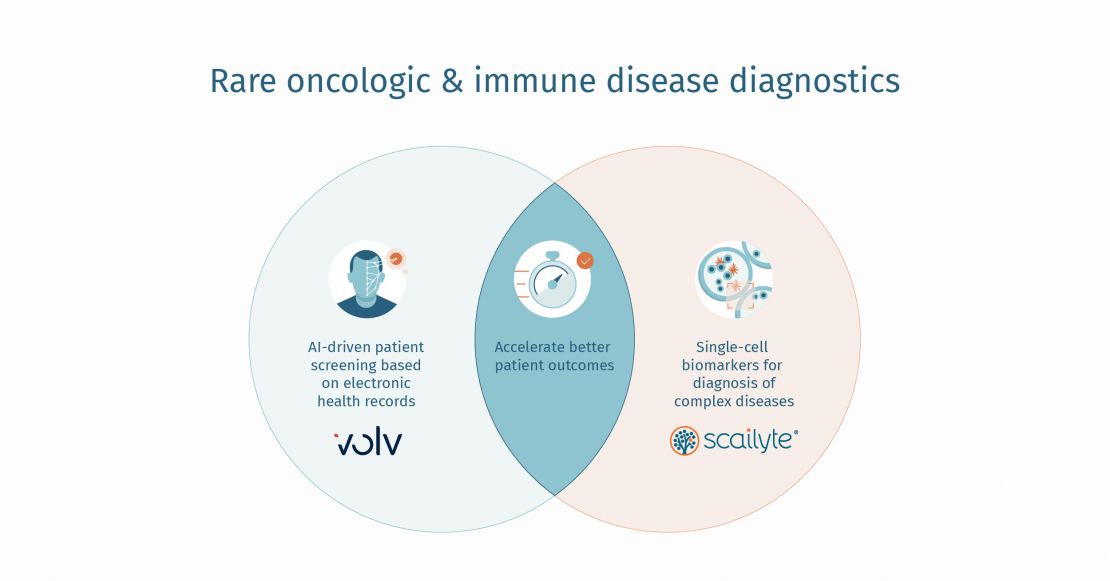
Rare disease drug developers face significant challenges during clinical development, from finding patients to conduct their trials to addressing heterogeneity in the target patient population. To help rare disease innovators establish a better clinical development strategy, Volv is co-conducting an in-depth Workshop at the World Orphan Drug Congress (WODC) Boston, USA, which is being held between the 11th to 13th July 2022. Come and meet us at Booth #318.
Join us at our Workshop on Monday 11th July 15:00 to discuss:
Putting AI to work for rare diseases: How can AI inform better Clinical Development strategy, design, and patient stratification?
The Workshop will consider AI’s potential with inClude, for revolutionising how companies operate in the rare disease space. Areas that will be explored include:
- novel approaches to obtaining new insights,
- uncovering new information from claims data,
- ways to better define target patient populations and novel endpoints, and
- gaining new insights into the disease earlier in its progression.
Download the Workshop Agenda here:
Creating a better clinical development strategy for rare diseases through AI innovation inClude.
The company’s most recent AI tool, inClude, helps sponsors identify the target patient population among both diagnosed and undiagnosed rare disease patients, establish clinical outcome assessments that characterise earlier disease states and can be considered as novel endpoints in clinical trials, and expand the range of biomarkers to advance biological understanding of the disease.
“If companies can find rare disease patients earlier, the symptoms those patients are experiencing might be different, which could help them develop a different and better target product profile and new outcome assessments with potentially differentiating endpoints,” says Christopher Rudolf, Founder and CEO of Volv Global SA. “Not only would this help to reduce risk in clinical development by providing greater understanding of the disease pathway, but it would also enable companies to differentiate their product.”
Join us at our Workshop on Monday 11th July 15:00 to discuss:
Putting AI to work for rare diseases: How can AI inform better Clinical Development strategy, design, and patient stratification?
The Workshop will include a joint presentation between Volv’s CEO Christopher Rudolf, Philipp von Gallwitz, Co-Founder and Managing Partner of admedicum, and Robert Pleticha,
Head of admedicum Spain, and International Patient Engagement Consultant.
Patient engagement is admedicum’s core focus and by listening to patients, these new endpoints can be validated and challenged with real patients. We see the value in working together to combine Volv’s data insights and admedicum’s validation of those insights with real people living with the disease.
During the Workshop, attendees will be split into 3 groups to discuss:
- how can identification of target patient populations be improved, and why is this needed?
- what new insights about the patient experience can inform new outcome assessment and potentially differentiating endpoints? And,
- which methods can help to improve the phenotyping of patients and lead to the identification of new biomarker candidates?
After which we will regroup, summarise the key points raised and hold a panel discussion to share the findings which post-congress will be presented in a White Paper and published on our Website.
As usual, we are expecting a lively and stimulating Workshop! We want to hear your ideas, no matter how idealistic they seem to you. Let's cast the net really wide and see what we can gather. We'll then add the capability.